Recently, MIT chemists have developed a new 3D printing technology that allows changing the chemical structure of printed objects and the chemical connections of multiple 3D printed objects. It is reported that this technology can greatly expand the complexity of objects created using 3D printing.
3D printing is an incredible manufacturing technique that creates many things from many materials. But the technology has limitations: on the one hand, 3D printed objects are generally immutable. They can be post-treated, sanded, and even processed into smaller shapes, but the chemical structure of 3D printed polymer objects is fixed. But now, a group of chemists at the Massachusetts Institute of Technology have developed new techniques for changing the chemical structure of 3D printed objects. The chemical composition can be changed after printing. This technology also allows multiple 3D printed objects to be fused together.
Now, the MIT team published their findings in the recent ACS Central Science Journal. Jeremiah Johnson is an associate professor of career development at the MIT chemistry program at Firmench and a senior author of research papers. He explains to MIT staff how to use this new technology to increase the complexity of 3D printed objects. “The idea is that you can print a material and then take it, use light to turn the material into something else, or grow the material further,†he said.
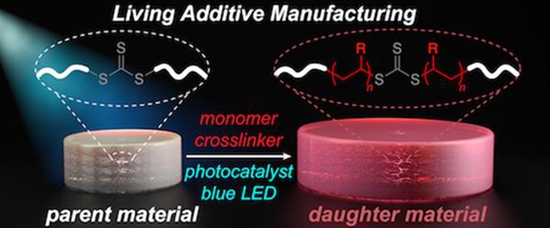
Stereolithography, 3D Systems' first liquid resin 3D printing technology, and liquid resin 3D printing technology promoted by companies such as Formlabs are one of the more accurate processes for ordinary users of 3D printing technology. A stereolithography 3D printer illuminates a series of bright projections onto a barrel of liquid resin that solidifies (hardens) in response to light, forming a solid object layer by layer. By using stereolithography and combining it with a technique called "living polymerization," Johnson and his team have been able to create 3D printed materials that can stop their growth and then start over at a later point in time.
As early as 2013, researchers at the Massachusetts Institute of Technology found that by using ultraviolet light, they can break down the polymer of the 3D printed structure and create reactive molecules called "free radicals." The free radicals can then bind to the surrounding new monomers and incorporate them into the original material. Johnson said: "The advantage here is that you can turn on the lights, they grow, you turn off the lights, they stop. In principle, you can repeat indefinitely, they can continue to grow."
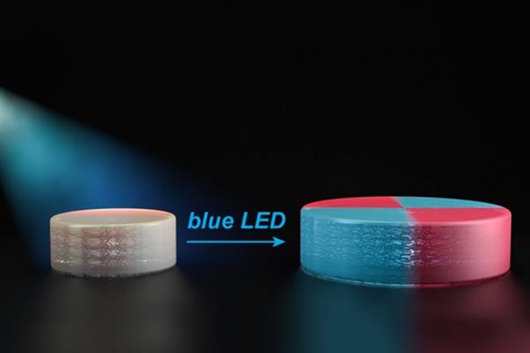
Unfortunately, attempts to control free radicals have proven to be very difficult, applying excessive damage to 3D printed materials. But MIT chemists have come up with another way: blue light from LEDs . Polymers such as those used for 3D printing contain the chemical group TTC, which can be activated by an organic catalyst that is opened by light. These TTCs stretch as new monomers adhere when exposed to blue light from the LEDs. Since these monomers are added evenly, they provide new properties to the material. “We can take macro materials and grow in the way we want,†Johnson said.
Using LED light technology, researchers at the Massachusetts Institute of Technology found that they can change the various properties of 3D printed object structures, including their stiffness and hydrophobicity (the extent to which they repel or absorb water). By adding some type of monomer, chemists can also swell or shrink the material in response to temperature. In addition, they are able to melt two 3D printed objects by illuminating the light on the interconnected areas. "This particular process can be used to create huge, chemically stable 3D printed structures with unprecedented complexity," the researchers said.
One of the obstacles faced by researchers today is to keep the experimental environment anaerobic because the organic catalyst used in the process does not work in the presence of oxygen. However, this set of tests can catalyze other catalysts similar to polymerization in an aerobic environment.
By combining polymer science and materials science, MIT researchers have opened up several exciting opportunities for advanced 3D printing.
For more LED related information, please click on the LED network or follow the WeChat public account (cnledw2013).
Oil Filter,Car Oil Filter,Automobile Oil Filter,Auto Oil Filter
Zhoushan Shenying Filter Manufacture Co., Ltd. , https://www.renkenfilter.com